A medication error is an unintended failure in the drug treatment process that leads to, or has the potential to lead to, harm to the patient. Mistakes in the prescribing, dispensing, storing, preparation and administration of a medicine are the most common preventable cause of undesired adverse events in medication practice and present a major public health burden. European Union (EU) legislation requires information on medication errors to be collected and reported through national pharmacovigilance systems. In addition, the European Medicines Agency (EMA) plays a coordinating role and has published a set of good practice guidance.
 |
| Medication errors in Pharmacovigilance |
Stages of Medication Errors
- Prescribing
- Omission
- Wrong time
- Unauthorized drug
- Improper dose
- Wrong dose prescription/wrong dose preparation
- Administration errors including the incorrect route of administration, giving the drug to the wrong patient, extra dose or wrong rate
- Monitoring error such as failing to take into account patient liver and renal function, failing to document allergy or potential for drug interaction
- Compliance error such as not following protocol or rules established for dispensing and prescribing medications
How do medication errors happen?
Medication errors can happen to anyone in any place, including your own home and at the doctor’s office, hospital, pharmacy and senior living facility. Kids are especially at high risk for medication errors because they typically need different drug doses than adults.
Knowing what you’re up against can help you play it safe. The most common causes of medication errors are:
- Poor communication between your doctors
- Poor communication between you and your doctors
- Drug names that sound alike and medications that look alike
- Medical abbreviations
Medication Error Risk Factors
- High volume
- Poor handwriting
- Inexperienced staff
- Challenging patient populations
- Lack of follow-up
- Lack of appropriate monitoring
- Lack of policy enforcement
- Medically complex patients
- Medications requiring calculations
- Environmental factors
- Poor communication
- Shift work
- Workplace culture
- Verbal orders
- Interpersonal factors such as external stress
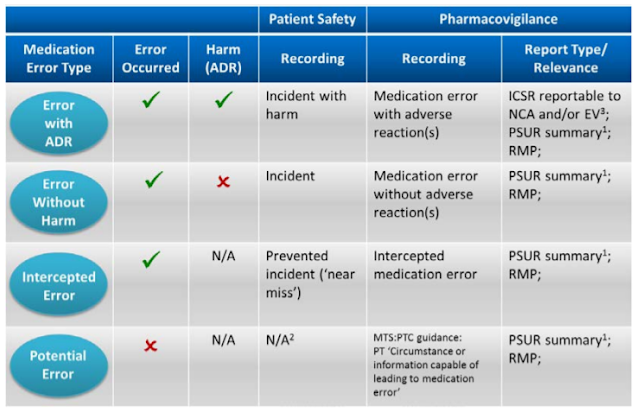
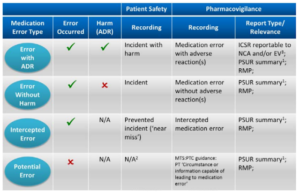
Intercepted medication error (‘near miss’)
In the context of pharmacovigilance an intercepted error indicates that an intervention caused a break in the chain of events in the treatment process before reaching the patient which would have resulted in a ‘potential’ ADR. The intervention has prevented actual harm being caused to the patient, e.g. a wrongly prepared medicine was actually not administered to the patient because the error was noticed by the nurse. In the context of patient safety reporting systems the term ‘near miss’ is used as synonym for describing what is classified ‘intercepted error’ for pharmacovigilance purposes. A near miss from a patient safety perspective is a random break in the chain of events leading up to a potential adverse event which has prevented injury, damage, illness or harm, but the potential for harm was nonetheless very near.
Potential medication error
According to GVP Module VII.B.5.9 a potential medication error is the recognition of circumstances that could lead to a medication error, and may or may not involve a patient. The term potential medication error refers to all possible mistakes in the prescribing, storing, dispensing, preparation for administration or administration of a medicinal product by all persons who are involved in the medication process and may lead to a) a medication error with harm, but without knowing the actual cause, b) a medication error without harm and without knowing the actual cause, or c) a medication error without harm, but with the awareness of the actual cause.
Following this approach, errors could be assigned to at least one of the above scenarios a, b or c with blurred borders between these scenarios.
Recording Medication Errors
Below Table shows the reporting requirements for marketing authorisation holders in line with EU pharmacovigilance obligations and relevant GVP recommendations.
 |
| Recording Medication Errors |
Strategies to Reduce Medication Errors
Over the years, hospitals have developed strategies to prevent medication errors. Some of these strategies include the following:
- Double-check the dosing and frequency of all high-alert medications. The Institute of Safe Medication Practices provides a list of high alert medications.
- If unsure about the drug or the dose, speak to the pharmacist.
- If the writing is illegible, do not give the medication believing that you think you know what it is. Call the healthcare provider to confirm the drug or dose.
- Recheck the calculation to ensure that the patient will get the right therapeutic dose.
- Ask another clinician to recheck your calculations.
Preventing Medication Errors
- Always write one prescription for each medication.
- Besides signing the prescription, always circle your name on the preprinted prescription pad.
- Do not hesitate to check the dose and frequency if you are not sure.
- Always consider the fact that each medication has the potential for adverse reactions.
- Do not use drug abbreviations when writing orders.
- Always add the patient’s age and weight to each prescription.
- Check for liver and renal function before ordering any medication.
- Spell out the frequency and route of dosage; do not use abbreviations.
- Always specify the duration of therapy; do not say give out “XXX” number of pills.
- Always be aware of high-risk medications.
- When writing a prescription, state the condition being treated.
A final word on medication errors
“Don’t ask, don’t tell” is never a smart policy when it comes to medications and your health. Don’t hesitate to ask questions or to tell your health care providers if anything seems amiss. Remember, you’re the final line of defense against medication errors.
If despite your efforts you have problems with a medication, talk with your doctor or pharmacist about whether to report it to MedWatch — the Food and Drug Administration safety and adverse event reporting program. Reporting to MedWatch is easy, confidential and secure — and it can help save others from being harmed by medication errors.
Regards,
PV Drug Safety Academy